what is happening with regard to intermolecular forces as a molecular liquid freezes
11.two: Intermolecular Forces
- Folio ID
- 21770
Learning Objectives
- To describe the intermolecular forces in liquids.
The properties of liquids are intermediate between those of gases and solids, just are more similar to solids. In contrast to intramolecular forces, such equally the covalent bonds that concord atoms together in molecules and polyatomic ions, intermolecular forces hold molecules together in a liquid or solid. Intermolecular forces are mostly much weaker than covalent bonds. For instance, it requires 927 kJ to overcome the intramolecular forces and break both O–H bonds in 1 mol of water, but it takes only well-nigh 41 kJ to overcome the intermolecular attractions and convert one mol of liquid h2o to water vapor at 100°C. (Despite this seemingly low value, the intermolecular forces in liquid water are amid the strongest such forces known!) Given the big difference in the strengths of intra- and intermolecular forces, changes between the solid, liquid, and gaseous states almost invariably occur for molecular substances without breaking covalent bonds.
The properties of liquids are intermediate between those of gases and solids, just are more similar to solids.
Intermolecular forces make up one's mind bulk properties, such every bit the melting points of solids and the boiling points of liquids. Liquids boil when the molecules accept enough thermal free energy to overcome the intermolecular bonny forces that hold them together, thereby forming bubbles of vapor within the liquid. Similarly, solids melt when the molecules learn enough thermal free energy to overcome the intermolecular forces that lock them into identify in the solid.
Intermolecular forces are electrostatic in nature; that is, they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds, intermolecular interactions are the sum of both attractive and repulsive components. Considering electrostatic interactions autumn off rapidly with increasing distance between molecules, intermolecular interactions are most important for solids and liquids, where the molecules are close together. These interactions become important for gases only at very high pressures, where they are responsible for the observed deviations from the ideal gas law at loftier pressures.
In this section, we explicitly consider three kinds of intermolecular interactions.There are two boosted types of electrostatic interaction that you are already familiar with: the ion–ion interactions that are responsible for ionic bonding, and the ion–dipole interactions that occur when ionic substances dissolve in a polar substance such equally water. The outset two are often described collectively as van der Waals forces.
Dipole–Dipole Interactions
Polar covalent bonds acquit as if the bonded atoms have localized fractional charges that are equal but contrary (i.e., the two bonded atoms generate a dipole). If the structure of a molecule is such that the individual bail dipoles exercise not cancel one another, so the molecule has a net dipole moment. Molecules with net dipole moments tend to align themselves so that the positive cease of one dipole is virtually the negative finish of another and vice versa, as shown in Figure \(\PageIndex{1a}\).
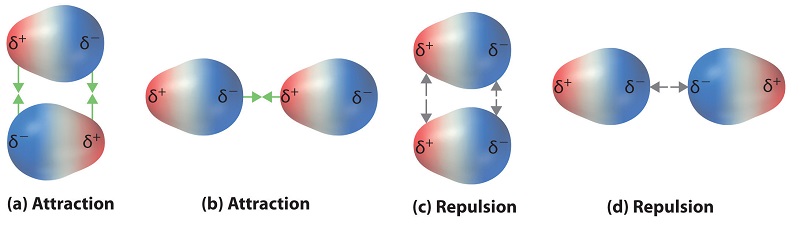
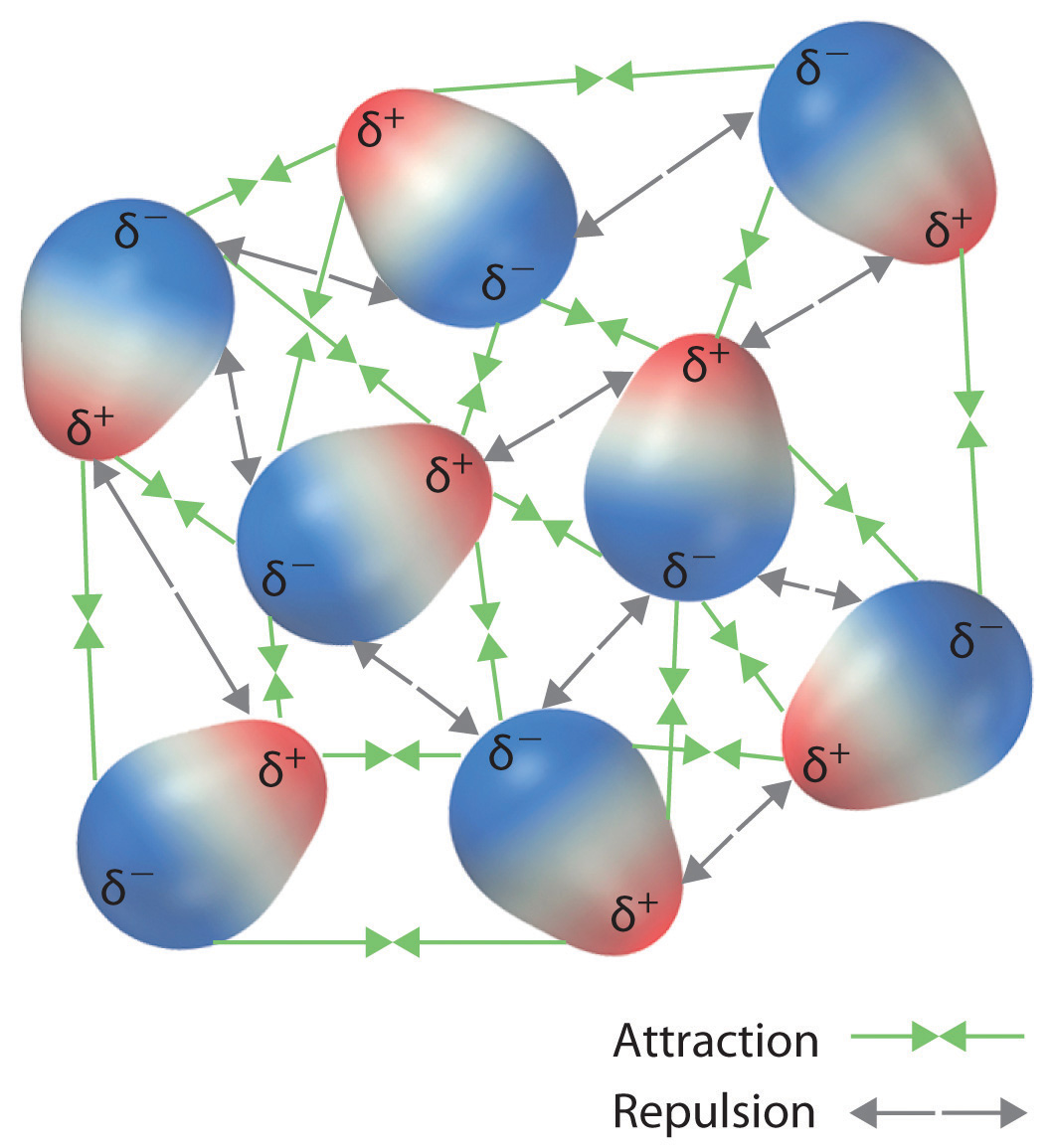
These arrangements are more stable than arrangements in which ii positive or two negative ends are next (Figure \(\PageIndex{1c}\)). Hence dipole–dipole interactions, such equally those in Effigy \(\PageIndex{1b}\), are attractive intermolecular interactions, whereas those in Figure \(\PageIndex{1d}\) are repulsive intermolecular interactions. Because molecules in a liquid motility freely and continuously, molecules always experience both attractive and repulsive dipole–dipole interactions simultaneously, equally shown in Figure \(\PageIndex{2}\). On average, however, the attractive interactions dominate.
Because each end of a dipole possesses but a fraction of the charge of an electron, dipole–dipole interactions are substantially weaker than the interactions between two ions, each of which has a charge of at to the lowest degree ±i, or betwixt a dipole and an ion, in which 1 of the species has at least a full positive or negative charge. In addition, the attractive interaction between dipoles falls off much more chop-chop with increasing distance than practice the ion–ion interactions. Recollect that the bonny energy betwixt two ions is proportional to 1/r, where r is the distance betwixt the ions. Doubling the distance (r → 2r) decreases the attractive energy past ane-half. In dissimilarity, the energy of the interaction of 2 dipoles is proportional to 1/r three, then doubling the altitude between the dipoles decreases the strength of the interaction by iiiii, or 8-fold. Thus a substance such equally \(\ce{HCl}\), which is partially held together by dipole–dipole interactions, is a gas at room temperature and 1 atm pressure. Conversely, \(\ce{NaCl}\), which is held together by interionic interactions, is a high-melting-betoken solid. Within a series of compounds of similar molar mass, the force of the intermolecular interactions increases every bit the dipole moment of the molecules increases, equally shown in Table \(\PageIndex{ane}\).
| Compound | Molar Mass (g/mol) | Dipole Moment (D) | Boiling Point (Thou) |
|---|---|---|---|
| C3Hsix (cyclopropane) | 42 | 0 | 240 |
| CH3OCH3 (dimethyl ether) | 46 | i.30 | 248 |
| CH3CN (acetonitrile) | 41 | 3.9 | 355 |
The attractive energy between ii ions is proportional to one/r, whereas the bonny free energy betwixt two dipoles is proportional to one/r6.
Video Discussing Dipole Intermolecular Forces. Source: https://youtu.exist/ACq_95SIBck
Example \(\PageIndex{1}\)
Adjust ethyl methyl ether (CH3OCHiiCH3), 2-methylpropane [isobutane, (CHthree)2CHCH3], and acetone (CH3COCHiii) in order of increasing boiling points. Their structures are as follows:
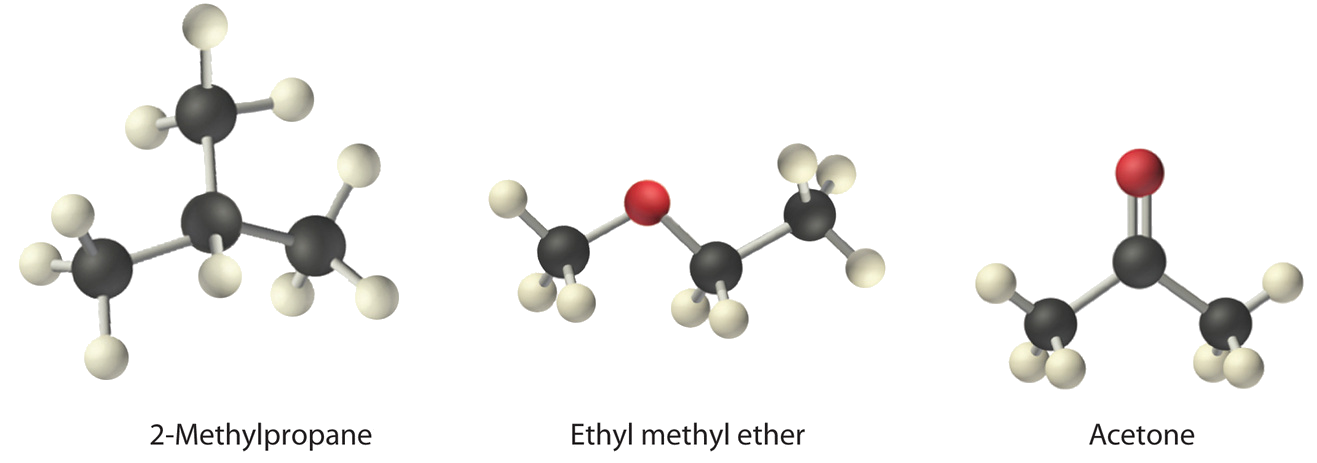
Given: compounds.
Asked for: social club of increasing humid points.
Strategy:
Compare the tooth masses and the polarities of the compounds. Compounds with higher tooth masses and that are polar volition have the highest humid points.
Solution:
The three compounds take substantially the aforementioned tooth mass (58–sixty m/mol), and then nosotros must look at differences in polarity to predict the force of the intermolecular dipole–dipole interactions and thus the boiling points of the compounds.
The showtime chemical compound, two-methylpropane, contains only C–H bonds, which are non very polar considering C and H have similar electronegativities. It should therefore have a very small (only nonzero) dipole moment and a very low boiling signal.
Ethyl methyl ether has a construction similar to H2O; information technology contains two polar C–O single bonds oriented at about a 109° angle to each other, in addition to relatively nonpolar C–H bonds. As a result, the C–O bond dipoles partially reinforce one another and generate a pregnant dipole moment that should give a moderately high humid point.
Acetone contains a polar C=O double bond oriented at well-nigh 120° to two methyl groups with nonpolar C–H bonds. The C–O bond dipole therefore corresponds to the molecular dipole, which should event in both a rather large dipole moment and a high boiling bespeak.
Thus we predict the following club of humid points:
2-methylpropane < ethyl methyl ether < acetone
This effect is in expert agreement with the actual data: ii-methylpropane, boiling point = −11.7°C, and the dipole moment (μ) = 0.xiii D; methyl ethyl ether, boiling point = vii.four°C and μ = 1.17 D; acetone, boiling bespeak = 56.1°C and μ = 2.88 D.
Practise \(\PageIndex{1}\)
Conform carbon tetrafluoride (CFfour), ethyl methyl sulfide (CHiiiSC2H5), dimethyl sulfoxide [(CH3)2Due south=O], and 2-methylbutane [isopentane, (CH3)twoCHCH2CH3] in club of decreasing boiling points.
- Answer
-
dimethyl sulfoxide (boiling point = 189.nine°C) > ethyl methyl sulfide (boiling point = 67°C) > 2-methylbutane (boiling point = 27.8°C) > carbon tetrafluoride (boiling point = −128°C)
London Dispersion Forces
Thus far, we have considered only interactions betwixt polar molecules. Other factors must be considered to explain why many nonpolar molecules, such equally bromine, benzene, and hexane, are liquids at room temperature; why others, such as iodine and naphthalene, are solids. Fifty-fifty the noble gases can be liquefied or solidified at low temperatures, high pressures, or both (Table \(\PageIndex{2}\)).
What kind of attractive forces tin be between nonpolar molecules or atoms? This question was answered by Fritz London (1900–1954), a High german physicist who after worked in the Us. In 1930, London proposed that temporary fluctuations in the electron distributions within atoms and nonpolar molecules could result in the formation of short-lived instantaneous dipole moments, which produce bonny forces called London dispersion forces betwixt otherwise nonpolar substances.
| Substance | Tooth Mass (m/mol) | Melting Point (°C) | Boiling Indicate (°C) |
|---|---|---|---|
| Ar | 40 | −189.iv | −185.nine |
| Xe | 131 | −111.8 | −108.1 |
| Northii | 28 | −210 | −195.8 |
| Otwo | 32 | −218.8 | −183.0 |
| F2 | 38 | −219.seven | −188.1 |
| Itwo | 254 | 113.seven | 184.4 |
| CH4 | 16 | −182.5 | −161.v |
Consider a pair of adjacent He atoms, for example. On average, the two electrons in each He cantlet are uniformly distributed around the nucleus. Because the electrons are in abiding motion, yet, their distribution in ane atom is likely to be asymmetrical at any given instant, resulting in an instantaneous dipole moment. Equally shown in part (a) in Figure \(\PageIndex{3}\), the instantaneous dipole moment on one atom can collaborate with the electrons in an next cantlet, pulling them toward the positive finish of the instantaneous dipole or repelling them from the negative end. The cyberspace effect is that the commencement cantlet causes the temporary formation of a dipole, called an induced dipole, in the second. Interactions between these temporary dipoles cause atoms to be attracted to ane another. These attractive interactions are weak and fall off chop-chop with increasing distance. London was able to show with quantum mechanics that the attractive energy between molecules due to temporary dipole–induced dipole interactions falls off as 1/r 6. Doubling the distance therefore decreases the attractive energy by 26, or 64-fold.
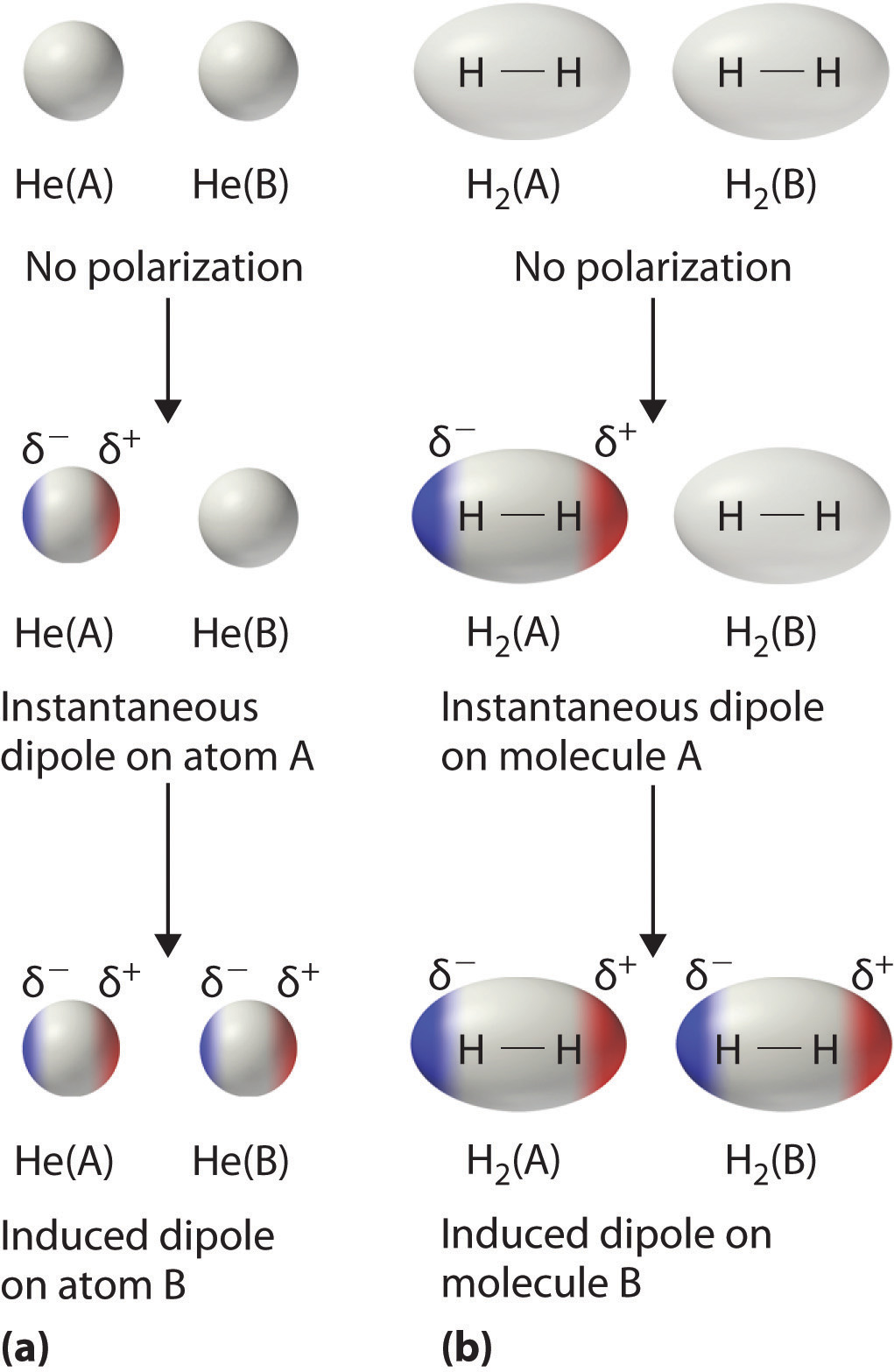
Instantaneous dipole–induced dipole interactions between nonpolar molecules can produce intermolecular attractions just as they produce interatomic attractions in monatomic substances similar Xe. This issue, illustrated for two Hii molecules in part (b) in Figure \(\PageIndex{3}\), tends to go more pronounced as atomic and molecular masses increment (Table \(\PageIndex{2}\)). For example, Xe boils at −108.1°C, whereas He boils at −269°C. The reason for this trend is that the forcefulness of London dispersion forces is related to the ease with which the electron distribution in a given atom can be perturbed. In small atoms such equally He, the two 1s electrons are held close to the nucleus in a very small book, and electron–electron repulsions are strong plenty to prevent meaning asymmetry in their distribution. In larger atoms such equally Xe, however, the outer electrons are much less strongly attracted to the nucleus because of filled intervening shells. As a result, it is relatively like shooting fish in a barrel to temporarily deform the electron distribution to generate an instantaneous or induced dipole. The ease of deformation of the electron distribution in an atom or molecule is called its polarizability. Because the electron distribution is more easily perturbed in large, heavy species than in small, low-cal species, we say that heavier substances tend to be much more polarizable than lighter ones.
For similar substances, London dispersion forces go stronger with increasing molecular size.
The polarizability of a substance also determines how information technology interacts with ions and species that possess permanent dipoles. Thus, London dispersion forces are responsible for the general tendency toward higher boiling points with increased molecular mass and greater surface area in a homologous series of compounds, such as the alkanes (office (a) in Figure \(\PageIndex{4}\)). The strengths of London dispersion forces also depend significantly on molecular shape because shape determines how much of one molecule can interact with its neighboring molecules at any given time. For example, part (b) in Figure \(\PageIndex{4}\) shows two,2-dimethylpropane (neopentane) and n-pentane, both of which have the empirical formula C5H12. Neopentane is almost spherical, with a small surface area for intermolecular interactions, whereas due north-pentane has an extended conformation that enables information technology to come into shut contact with other n-pentane molecules. As a result, the boiling point of neopentane (nine.5°C) is more than 25°C lower than the boiling signal of n-pentane (36.1°C).
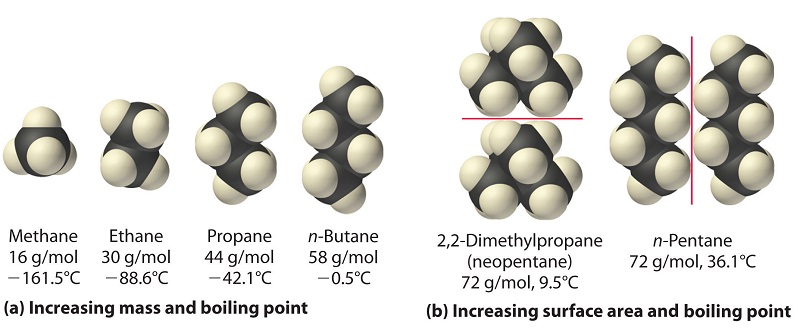
All molecules, whether polar or nonpolar, are attracted to ane another by London dispersion forces in improver to whatsoever other attractive forces that may exist present. In general, all the same, dipole–dipole interactions in minor polar molecules are significantly stronger than London dispersion forces, and so the former predominate.
Video Discussing London/Dispersion Intermolecular Forces. Source: https://youtu.be/RCRTcIEQ-Hk
Example \(\PageIndex{2}\)
Suit n-butane, propane, 2-methylpropane [isobutene, (CH3)2CHCH3], and n-pentane in order of increasing boiling points.
Given: compounds
Asked for: lodge of increasing boiling points
Strategy:
Determine the intermolecular forces in the compounds, and so arrange the compounds according to the strength of those forces. The substance with the weakest forces will have the everyman boiling point.
Solution:
The iv compounds are alkanes and nonpolar, so London dispersion forces are the only important intermolecular forces. These forces are generally stronger with increasing molecular mass, so propane should have the everyman boiling point and due north-pentane should accept the highest, with the two butane isomers falling in between. Of the ii butane isomers, 2-methylpropane is more than meaty, and n-butane has the more than extended shape. Consequently, we expect intermolecular interactions for northward-butane to exist stronger due to its larger surface area, resulting in a higher boiling point. The overall order is thus as follows, with actual humid points in parentheses: propane (−42.1°C) < two-methylpropane (−eleven.7°C) < n-butane (−0.v°C) < n-pentane (36.1°C).
Practise \(\PageIndex{2}\)
Arrange GeHiv, SiCl4, SiH4, CH4, and GeClfour in order of decreasing humid points.
- Answer
-
GeCl4 (87°C) > SiCl4 (57.vi°C) > GeH4 (−88.5°C) > SiH4 (−111.viii°C) > CHiv (−161°C)
Hydrogen Bonds
Molecules with hydrogen atoms bonded to electronegative atoms such as O, N, and F (and to a much lesser extent, Cl and Southward) tend to exhibit unusually strong intermolecular interactions. These effect in much higher boiling points than are observed for substances in which London dispersion forces dominate, as illustrated for the covalent hydrides of elements of groups fourteen–17 in Figure \(\PageIndex{5}\). Methane and its heavier congeners in grouping 14 course a series whose boiling points increase smoothly with increasing molar mass. This is the expected trend in nonpolar molecules, for which London dispersion forces are the exclusive intermolecular forces. In contrast, the hydrides of the lightest members of groups 15–17 have boiling points that are more than 100°C greater than predicted on the footing of their molar masses. The issue is near dramatic for h2o: if we extend the straight line connecting the points for H2Te and H2Se to the line for period ii, we obtain an estimated humid bespeak of −130°C for water! Imagine the implications for life on Earth if h2o boiled at −130°C rather than 100°C.
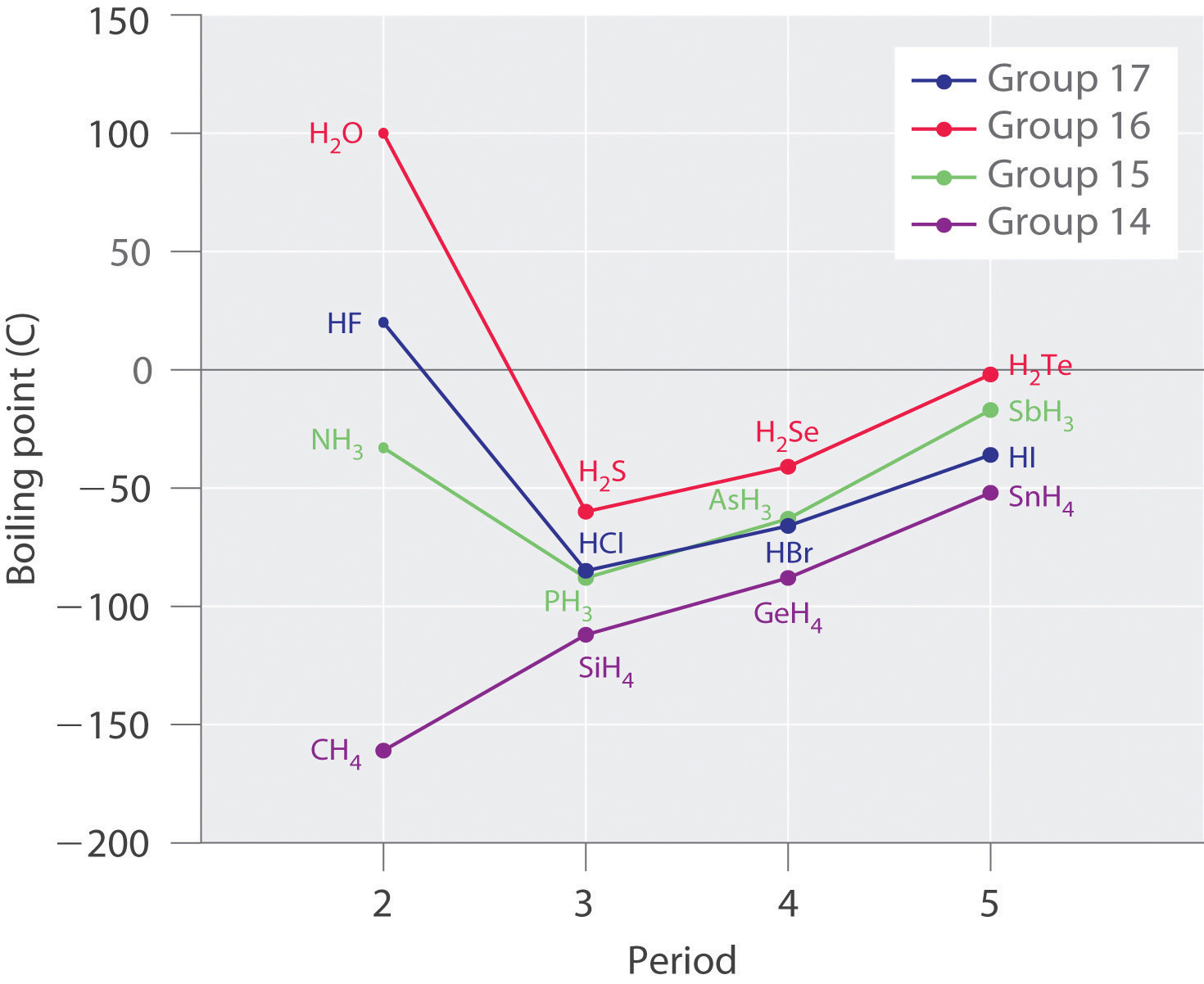
Why do potent intermolecular forces produce such anomalously high boiling points and other unusual properties, such every bit loftier enthalpies of vaporization and high melting points? The respond lies in the highly polar nature of the bonds between hydrogen and very electronegative elements such as O, N, and F. The big deviation in electronegativity results in a big partial positive charge on hydrogen and a correspondingly big partial negative charge on the O, N, or F cantlet. Consequently, H–O, H–N, and H–F bonds have very large bond dipoles that can interact strongly with one another. Because a hydrogen atom is then small, these dipoles can also approach i another more closely than near other dipoles. The combination of large bond dipoles and brusk dipole–dipole distances results in very strong dipole–dipole interactions called hydrogen bonds, every bit shown for water ice in Figure \(\PageIndex{half dozen}\). A hydrogen bond is usually indicated by a dotted line between the hydrogen atom attached to O, N, or F (the hydrogen bail donor) and the atom that has the lone pair of electrons (the hydrogen bond acceptor). Because each water molecule contains 2 hydrogen atoms and two lone pairs, a tetrahedral organisation maximizes the number of hydrogen bonds that tin can exist formed. In the structure of ice, each oxygen cantlet is surrounded past a distorted tetrahedron of hydrogen atoms that form bridges to the oxygen atoms of next water molecules. The bridging hydrogen atoms are not equidistant from the two oxygen atoms they connect, notwithstanding. Instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. In contrast, each oxygen atom is bonded to ii H atoms at the shorter distance and two at the longer distance, corresponding to two O–H covalent bonds and 2 O⋅⋅⋅H hydrogen bonds from adjacent h2o molecules, respectively. The resulting open, cagelike structure of water ice means that the solid is actually slightly less dense than the liquid, which explains why water ice floats on water, rather than sinks.
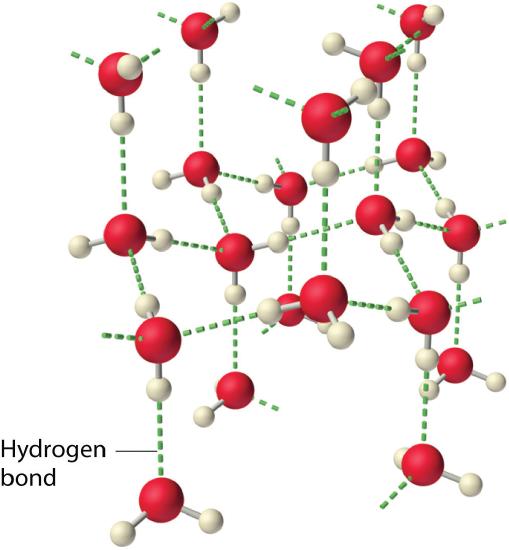
Each h2o molecule accepts ii hydrogen bonds from ii other water molecules and donates two hydrogen atoms to form hydrogen bonds with ii more h2o molecules, producing an open, cagelike structure. The construction of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion.
Hydrogen bond germination requires both a hydrogen bail donor and a hydrogen bond acceptor.
Because ice is less dumbo than liquid water, rivers, lakes, and oceans freeze from the top down. In fact, the water ice forms a protective surface layer that insulates the rest of the water, allowing fish and other organisms to survive in the lower levels of a frozen lake or body of water. If water ice were denser than the liquid, the ice formed at the surface in common cold atmospheric condition would sink as fast every bit it formed. Bodies of water would freeze from the bottom up, which would be lethal for near aquatic creatures. The expansion of water when freezing also explains why automobile or boat engines must be protected past "antifreeze" and why unprotected pipes in houses pause if they are allowed to freeze.
Video Discussing Hydrogen Bonding Intermolecular Forces. Source: https://youtu.be/92rbjSpHbr0
Example \(\PageIndex{3}\)
Considering CH3OH, C2H6, Xe, and (CH3)3N, which tin form hydrogen bonds with themselves? Draw the hydrogen-bonded structures.
Given: compounds
Asked for: formation of hydrogen bonds and structure
Strategy:
- Identify the compounds with a hydrogen cantlet attached to O, Northward, or F. These are likely to be able to act as hydrogen bond donors.
- Of the compounds that tin act as hydrogen bail donors, identify those that besides comprise solitary pairs of electrons, which allow them to be hydrogen bond acceptors. If a substance is both a hydrogen donor and a hydrogen bond acceptor, depict a construction showing the hydrogen bonding.
Solution:
A. Of the species listed, xenon (Xe), ethane (CiiHhalf dozen), and trimethylamine [(CH3)3N] do not contain a hydrogen atom attached to O, N, or F; hence they cannot human action every bit hydrogen bond donors.
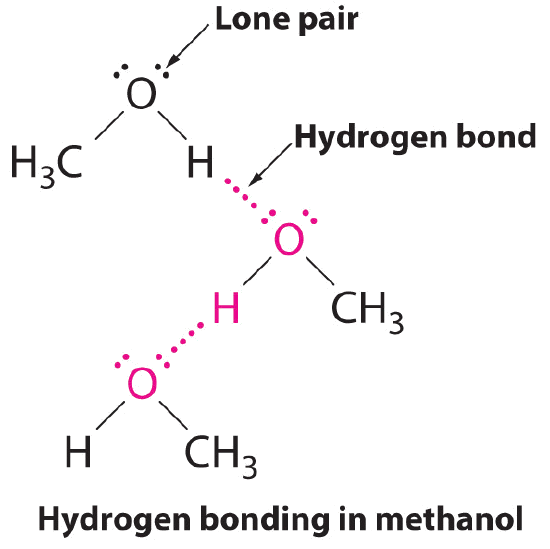
B. The ane compound that tin can human activity as a hydrogen bail donor, methanol (CHiiiOH), contains both a hydrogen cantlet attached to O (making it a hydrogen bond donor) and two alone pairs of electrons on O (making it a hydrogen bond acceptor); methanol tin can thus form hydrogen bonds by acting as either a hydrogen bond donor or a hydrogen bail acceptor. The hydrogen-bonded structure of methanol is every bit follows:
Exercise \(\PageIndex{3}\)
Because CH3COtwoH, (CH3)iiiDue north, NH3, and CHiiiF, which can form hydrogen bonds with themselves? Draw the hydrogen-bonded structures.
- Answer
-
CHthreeCO2H and NH3;

Although hydrogen bonds are significantly weaker than covalent bonds, with typical dissociation energies of only 15–25 kJ/mol, they have a significant influence on the physical properties of a compound. Compounds such equally HF can form just 2 hydrogen bonds at a time as can, on boilerplate, pure liquid NHiii. Consequently, fifty-fifty though their molecular masses are similar to that of water, their boiling points are significantly lower than the boiling indicate of water, which forms four hydrogen bonds at a time.
Example \(\PageIndex{four}\): Buckyballs
Arrange C60 (buckminsterfullerene, which has a cage structure), NaCl, He, Ar, and Due north2O in social club of increasing boiling points.
Given: compounds.
Asked for: order of increasing boiling points.
Strategy:
Identify the intermolecular forces in each compound and and so arrange the compounds according to the strength of those forces. The substance with the weakest forces will take the everyman boiling point.
Solution:
Electrostatic interactions are strongest for an ionic compound, so we expect NaCl to have the highest humid point. To predict the relative boiling points of the other compounds, we must consider their polarity (for dipole–dipole interactions), their ability to form hydrogen bonds, and their tooth mass (for London dispersion forces). Helium is nonpolar and past far the lightest, so it should accept the everyman humid point. Argon and N2O have very like molar masses (40 and 44 g/mol, respectively), but Due north2O is polar while Ar is not. Consequently, N2O should accept a college humid point. A C60 molecule is nonpolar, but its molar mass is 720 chiliad/mol, much greater than that of Ar or Due north2O. Because the humid points of nonpolar substances increase rapidly with molecular mass, Csixty should boil at a college temperature than the other nonionic substances. The predicted order is thus every bit follows, with actual humid points in parentheses:
He (−269°C) < Ar (−185.7°C) < NorthiiO (−88.five°C) < C60 (>280°C) < NaCl (1465°C).
Practise \(\PageIndex{4}\)
Arrange two,iv-dimethylheptane, Ne, CS2, Clii, and KBr in order of decreasing humid points.
- Answer
-
KBr (1435°C) > 2,four-dimethylheptane (132.9°C) > CSii (46.half dozen°C) > Cl2 (−34.6°C) > Ne (−246°C)
Example \(\PageIndex{5}\):
Identify the most meaning intermolecular forcefulness in each substance.
- C 3 H 8
- CH 3 OH
- H 2 S
Solution
a. Although C–H bonds are polar, they are only minimally polar. The about significant intermolecular force for this substance would exist dispersion forces.
b. This molecule has an H atom bonded to an O atom, and then it will experience hydrogen bonding.
c. Although this molecule does not experience hydrogen bonding, the Lewis electron dot diagram and VSEPR point that it is bent, so information technology has a permanent dipole. The almost meaning force in this substance is dipole-dipole interaction.
Exercise \(\PageIndex{6}\)
Identify the nearly meaning intermolecular strength in each substance.
- HF
- HCl
- Answer a
-
hydrogen bonding
- Answer b
-
dipole-dipole interactions
Summary
Intermolecular forces are electrostatic in nature and include van der Waals forces and hydrogen bonds. Molecules in liquids are held to other molecules past intermolecular interactions, which are weaker than the intramolecular interactions that agree the atoms together within molecules and polyatomic ions. Transitions between the solid and liquid, or the liquid and gas phases, are due to changes in intermolecular interactions, but practise non bear upon intramolecular interactions. The three major types of intermolecular interactions are dipole–dipole interactions, London dispersion forces (these 2 are frequently referred to collectively as van der Waals forces), and hydrogen bonds. Dipole–dipole interactions ascend from the electrostatic interactions of the positive and negative ends of molecules with permanent dipole moments; their forcefulness is proportional to the magnitude of the dipole moment and to i/r3 , where r is the distance between dipoles. London dispersion forces are due to the formation of instantaneous dipole moments in polar or nonpolar molecules equally a result of curt-lived fluctuations of electron charge distribution, which in turn crusade the temporary germination of an induced dipole in adjacent molecules; their energy falls off as 1/r 6. Larger atoms tend to exist more polarizable than smaller ones, because their outer electrons are less tightly bound and are therefore more easily perturbed. Hydrogen bonds are especially strong dipole–dipole interactions betwixt molecules that have hydrogen bonded to a highly electronegative atom, such as O, North, or F. The resulting partially positively charged H cantlet on ane molecule (the hydrogen bond donor) can interact strongly with a lonely pair of electrons of a partially negatively charged O, Northward, or F atom on adjacent molecules (the hydrogen bond acceptor). Because of strong O⋅⋅⋅H hydrogen bonding betwixt h2o molecules, h2o has an unusually loftier humid point, and ice has an open, cagelike structure that is less dense than liquid h2o.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_Chemistry_-_The_Central_Science_%28Brown_et_al.%29/11:_Liquids_and_Intermolecular_Forces/11.2:_Intermolecular_Forces
0 Response to "what is happening with regard to intermolecular forces as a molecular liquid freezes"
Post a Comment